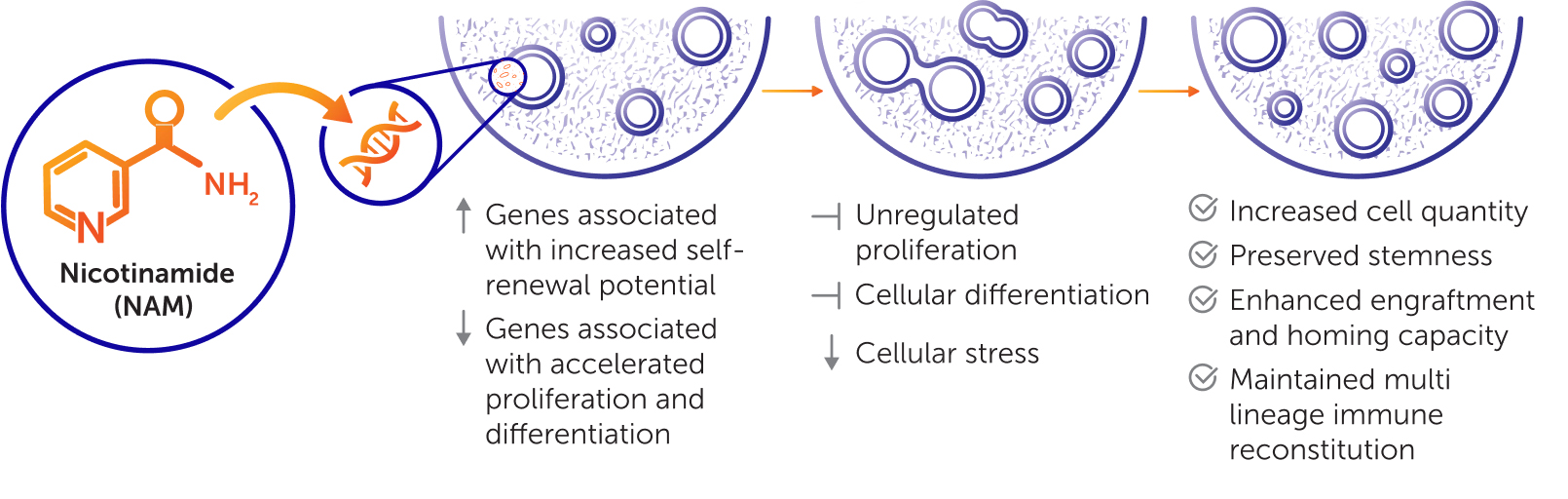
Nicotinamide (NAM) Technology
Omisirge is the first and only FDA-approved, NAM-modified allogeneic hematopoietic progenitor cell (HPC) therapy for patients 12 years and older with hematologic malignancies in need of transplant
Click on the blue plus signs for more information