Efficacy and Safety
Omisirge was evaluated in a global, randomized phase 3 trial across a diverse population of patients
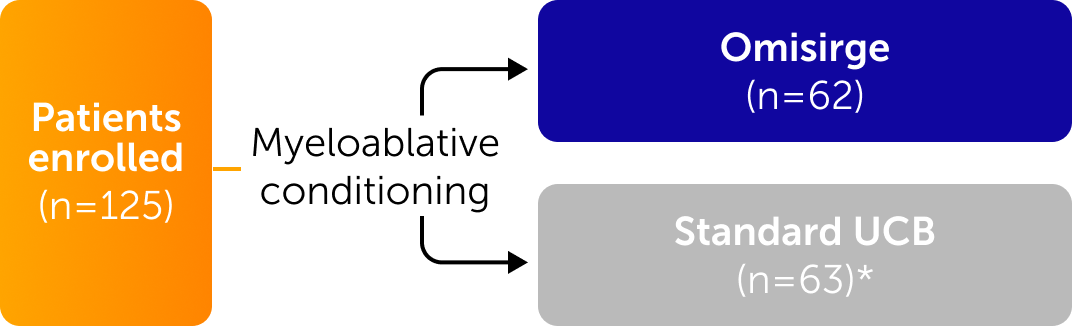
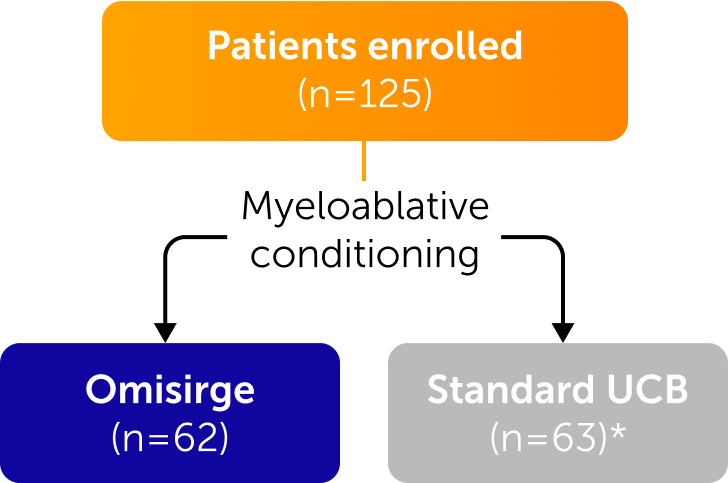
Inclusion Criteria
- Age 12-65 years
- Hematologic malignancies
- Eligible for allo-HCT
- No readily available matched related or matched unrelated adult donor
Patients who had haploidentical related donors or syngeneic donors were not excluded.
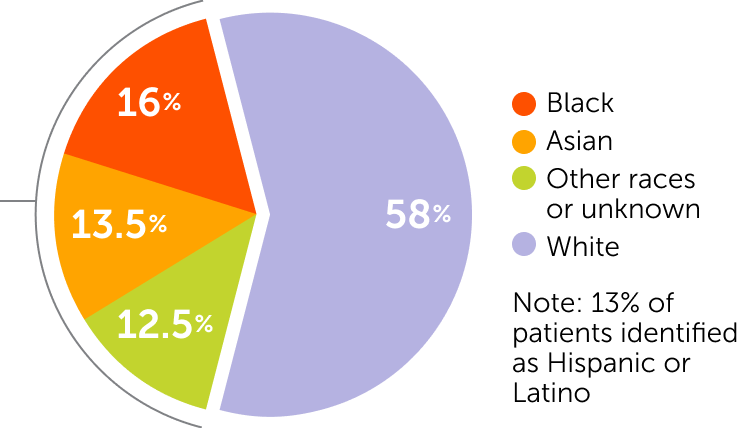
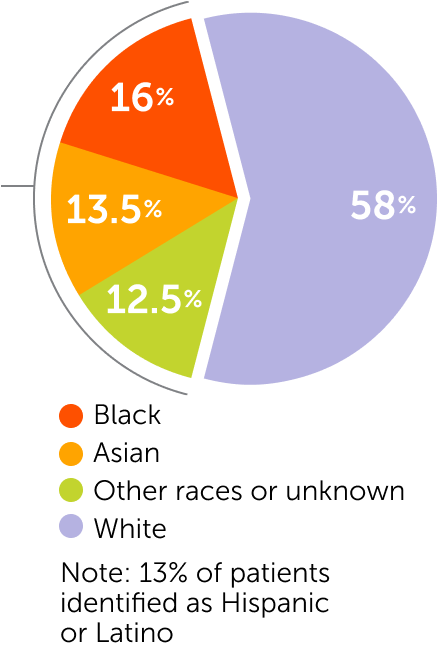
Patient Demographics
of patients enrolled were
from racially and ethnically
diverse backgrounds
Diagnosis
- AML (48%)
- ALL (33%)
- MDS (7%)
- CML (5%)
- Lymphoma (4%)
- Rare leukemias (3%)
Risk Index
- Moderate (42%)
- High / Very high (34%)
HCT-CI*
- ≥ 3 (50%)
- 1-2 (30%)
* HCT-CI: Hemtopoietic cell transplantation-specific comorbidity index
Trial endpoints were selected for their significance to patient outcomes
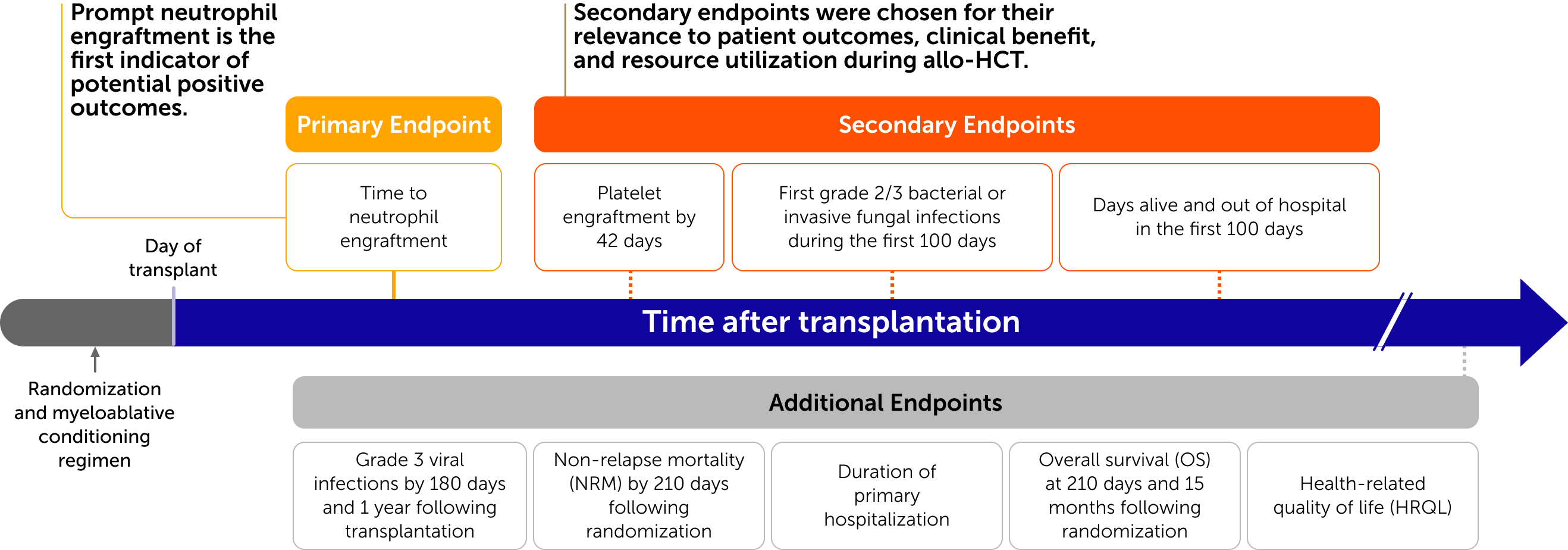
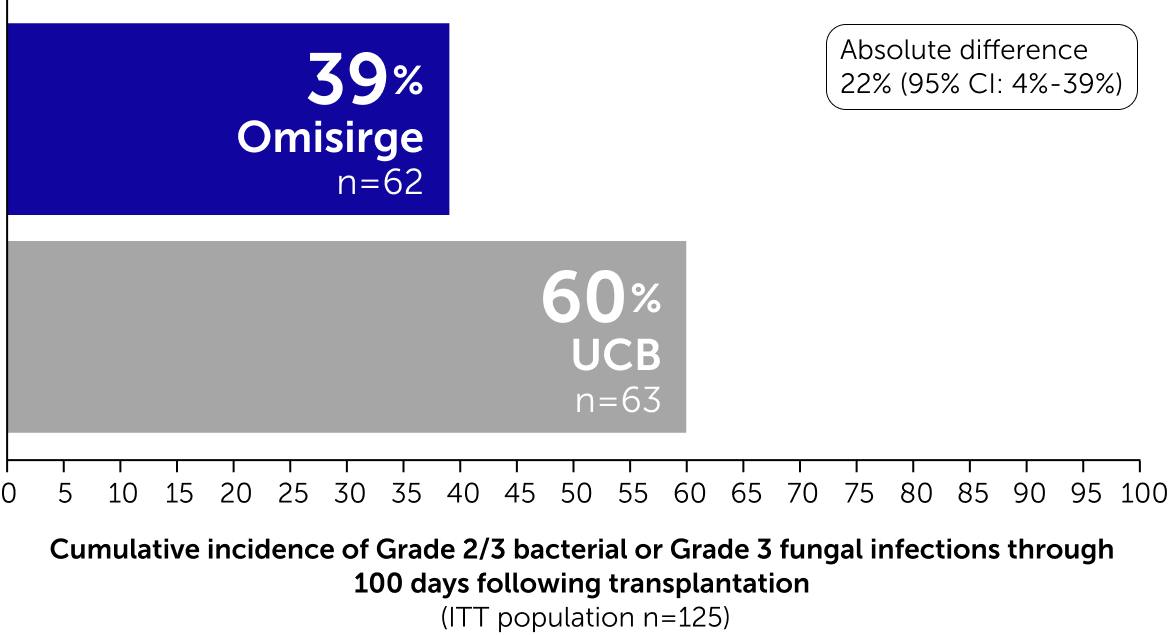
Omisirge demonstrated faster hematopoietic recovery with fewer infections
Patients transplanted with Omisirge experienced faster neutrophil recovery compared to those in the UCB control group (primary endpoint)


Patients transplanted with Omisirge had fewer bacterial and fungal infections after transplantation (secondary endpoint)

* Time to neutrophil recovery was defined as the time from transplantation to the earliest of 3 consecutive measurements on different days with absolute neutrophil counter greater than or equal to 0.5 GI/L assessed with 42 days of follow-up.
† Median time to neutrophil recovery was estimated by the Kaplan-Meier estimator.
Differences between the Omisirge and UCB arms for the Neutrophil Recovery and Bacterial and Fungal Infections endpoints were statistically significant.
Click to see additional endpoints evaluated in the phase 3 trial
Secondary Endpoints
Additional Endpoints
Swipe to see additional endpoints evaluated in the phase 3 trial
Omisirge has an established safety profile consistent with the expected adverse events of allo-HCT following conditioning therapy
CTCAE ≥Grade 3 adverse reactions in ≥10% of patients following transplantation with Omisirge or UCB (PP)
| Adverse Events | Omisirge, n=52 (%) | UCB, n=56 (%) |
|---|---|---|
| General disorders and administration site conditions | ||
| Pain* | 33 | 18 |
| Mucosal inflammation*† | 31 | 34 |
| Fatigue† | 4 | 21 |
| Fever | 2 | 11 |
| Vascular disorders | ||
| Hypertension*† | 25 | 38 |
| Hemorrhage | 12 | 18 |
| Gastrointestinal disorders | ||
| Gastrointestinal toxicity*† | 19 | 34 |
| Dysphagia | 12 | 13 |
| Renal and urinary disorders | ||
| Renal impairment | 12 | 5 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Respiratory failure† | 12 | 30 |
| Dyspnea | 8 | 16 |
* Most common Grade 3-5 AEs for Omisirge
† Most common Grade 3-5 AEs for UCB
Chemistry laboratory abnormalities in ≥10% of patients (PP)
| Laboratory Abnormalities | Omisirge, n=52 (%) | UCB, n=56 (%) | ||
|---|---|---|---|---|
| Grade 1-4 | Grade 3-4 | Grade 1-4 | Grade 3-4 | |
| Magnesium decreased | 94 | 4 | 91 | 2 |
| Aspartate aminotransferase increased | 56 | 13 | 61 | 7 |
| Alanine aminotransferase increased | 56 | 13 | 57 | 9 |
| Creatinine increased | 50 | 4 | 57 | 2 |
| Bilirubin increased | 42 | 12 | 61 | 21 |
| Alkaline phosphatase increased | 42 | 0 | 54 | 2 |
| Magnesium increased | 15 | 2 | 29 | 9 |
* Most common Grade 3-5 AEs for Omisirge
† Most common Grade 3-5 AEs for UCB
Infections following transplantation with Omisirge or UCB (PP)
| Infections | Omisirge, n=52 (%) | UCB, n=56 (%) |
||||
|---|---|---|---|---|---|---|
| Grade 1-3 | Grade 2 | Grade 3 | Grade 1-3 | Grade 2 | Grade 3 | |
| Grade | 1-3 | 2 | 3 | 1-3 | 2 | 3 |
| Viral | 75 | 48 | 8 | 80 | 32 | 27 |
| Bacterial | 65 | 27 | 8 | 80 | 46 | 23 |
| Fungal | 21 | 4 | 6 | 27 | 0 | 18 |
Acute and Chronic GvHD post treatment (PP)
| GvHD | Omisirge, n=52 (%) | UCB, n=56 (%) |
|---|---|---|
| Grade II-IV acute GvHD | 62 | 43 |
| Grade III-IV acute GvHD | 15 | 21 |
| Chronic GvHD | 35 | 25 |
| Moderate to severe chronic GvHD | 23 | 20 |
All patients received myeloablative preparative regimens and GvHD prophylaxis with tacrolimus or cyclosporin plus mycophenolate.

